Sorkin Lab

OUR RESEARCH
Some of our current and recent projects:
Migrasome formation
Migrasomes are a recently discovered type of extra-cellular vesicles generated from retraction fibers during cell migration on extra-cellular substrates. These vesicles, of several microns in size, allow cells to release contents at specific locations, which can be taken up by other cells which travel to that site. In this project, we combine micropipette aspiration, dual trap optical tweezers and confocal fluorescence microscopy to reveal the physico-chemical foundations of migrasome formation.
Want to know more? Read here:
We discovered that migrasomes form in a two-stage process, where the first step is initiated by mechanical forces and the second step of migrasome growth and stabilization is facilitated by TSPAN enrichment.
We demonstrated that tubular junctions are more susceptible to the pearling transition than tubules, explaining the preferential formation of migrasomes at the junctions of retraction fibers. LINK


Membrane fusion in fertilization and viral infection
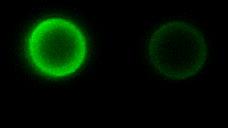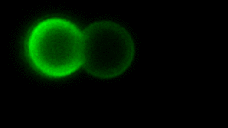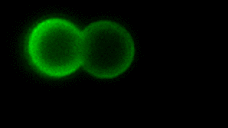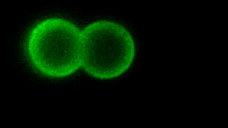
As the complexity of the cell membrane is very large and the roles of different components (e.g., thousands of different lipids and proteins) are difficult to disentangle, model systems with well-defined composition, the complexity of which can be gradually increased in a controlled manner, are instrumental to advance our understanding of membrane remodeling. We use well-controlled model systems in the form of micro-beads coated with membranes of defined composition, with attached/incorporated proteins of interest, to study protein-protein and protein-membrane interactions in membrane fusion. On the right, a short video demonstrates an optical tweezers measurement, where two beads are brought into contact and interaction forces are measured. Lipids and proteins can be labeled and visualized using confocal fluorescence microscopy, as shown in the gif:
Want to know more? Read here:
We have developed a method to coat microscopic beads with natural membranes, in order to study protein-protein interactions with optical tweezers:
We used proteins tethered to DNA linkers to measure unbinding forces between the Ebola virus fusion protein and a target membrane:

Role of tension and curvature in membrane shaping and remodeling events
Membrane tension and curvature play an important role in membrane associated processes. We want to understand the roles of these parameters in processes like membrane fusion. For example, we found that membrane tension inhibits hemifusion, an intermediate step in the fusion reaction, because it increases the energy barrier of the process:

Multiple membrane-shaping and remodeling processes are associated with tetraspanin proteins by unclear mechanisms. We revealed that tetraspanin4 and CD9 are curvature sensors with a preference for positive membrane curvature. To investigate this, we employed a biomimetic system that emulates membranes of cell retraction fibers and egg-cell microvilli. This system consists of membrane tubes pulled from giant plasma membrane vesicles, allowing for precise control of membrane tension and curvature.
Using this method on mutated proteins, we further discovered which parts of the tetraspanin protein are important for curvature sensitivity and lateral interactions.

Past project: Cell Mechanics
Mechanical properties of cells are important reporters of a cell's condition, as they often change upon disease. We have developed a new approach to measure mechanical properties of cells using acoustically exerted forces. This method allowed us to reveal that red blood cells become more deformable when they uptake extracellular vesicles.
Read popular science story here:
https://www.hfsp.org/hfsp-news-events/using-sound-stretch-cells
And manuscript here:





